Heat flux transducer: sensors and measurements
What most people call heat flux sensors are officially called heat flux transducers. In this article, we will first explain the difference between a heat flux sensor and a heat flux transducer. Second, we highlight the calorimeter; this is the simplest device that can be used to measure heat flux. We explain how a calorimeter functions and how it is made. Finally, the calorimeter is compared to more advanced thermopile-based heat flux sensors. We discuss the advantages and limitations of both.
What is a heat flux transducer?
The International Vocabulary of Metrology (VIM) is a document that gives definitions of concepts used in metrology (the science of measurements). In the VIM, we find the following definitions for sensor and for measuring transducer:
“Sensor; element of a measuring system that is directly affected by a phenomenon, body or substance carrying a quantity to be measured”.
“Sensor” is a very wide term. If we use temperature as a phenomenon, then everything that reacts to temperature can be considered a sensor. The Mercury inside a thermometer is a sensor, since it reacts to temperature change. But a table also reacts to temperature change (on a microscopic level) and can technically also be considered a sensor.
A measuring transducer is defined as follows:
“Measuring transducer; device, used in measurement, that provides an output quantity having a specified relation to the input quantity”.
The key concept here is “specified relation”. This is what separates a transducer from a sensor. The sensor simply reacts to an input quantity, nothing else. The measuring transducer responds to the input quantity and provides an output signal with a known relation to the input.
Returning to the thermometer; the Mercury alone gives us zero knowledge about the temperature. Mercury expands when the temperature rises, but you cannot know the temperature by looking at a pool of Mercury lying on a table.
We can make a thermometer by putting the Mercury inside a glass tube with markings at regular intervals. This allows us to see the exact change in volume when the temperature changes. Now all that’s left is the calibration process. The calibration process provides us with information on which temperature corresponds to each marking on the tube. The final product is a transducer that reacts to a temperature and allows us to see how high this temperature is by looking at the labelled markings.
Heat flux transducers and their measurement methods
What are the units of heat flux?
Heat transfer and heat flux are closely related to each other. Heat transfer is the total amount of heat (or energy) transferred to or from a material, using the unit Joule [J].
Heat flux is the heat transferred through a surface over a period of time, for which we use the symbol: ϕ. The units of heat flux are Joule/second/square meter [J⁄(m2s)] which we usually write as [W⁄m2] since one Watt [W] equals one Joule/second [J/s]. More on this in our article: What is heat flux.
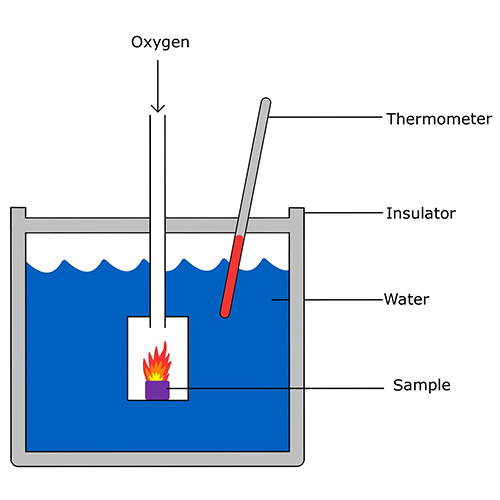
of calories in a biomass sample.
We will now discuss two different types of heat flux transducers that can be used to measure heat flux. One type is the calorimeter, and the other is the thermopile-based heat flux transducer.
What is a calorimeter?
Calorimeters are transducers used to measure absorbed or expelled heat in a chemical, mechanical or electrical process. This is called calorimetry. One calorie is the amount of energy needed to raise the temperature of 1 gram of water by 1 °C (or 1 K), equal to 4.184 J.
Figure 1 shows a simplified bomb calorimeter, used to measure the amount of calories in a given quantity of biomass. The heat transferred from the sample to the water allows us to calculate this amount of calories by using a simple formula: Cal = m·cs·ΔT, where m is the mass, cs is the specific heat capacity, and ΔT is the temperature change of water in kg, J/kg/K and K respectively. You can also measure how much mass the sample has lost while burning, making it possible to calculate the number of calories per unit mass, as seen on food packaging.
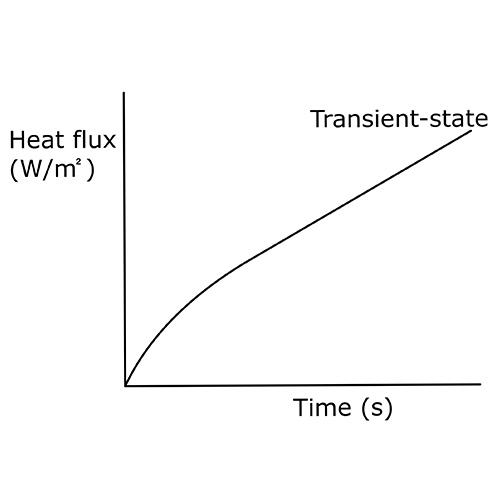
time and transient state heat flux.
The calorimeter measures what we call “transient-state heat flux”, shown in the graph in Figure 2. This means that the heat flux changes over time. Calorimeters can only be used for relatively short-time-interval measurements compared to the thermopile-based heat flux sensors. This is due to the fact that transient-state heat flux turns into steady-state when given enough time.
Measuring heat flux with a calorimeter
Another type of calorimeter, called a slug calorimeter, can be seen in Figure 3. This type of calorimeter is used to measure radiative heat flux. The “slug” is a piece of material (often copper) with a high heat capacity that absorbs radiation due to the black absorbing coating. We can assume that the slug does not lose any heat due to the insulator. A thermocouple within the slug is used to measure its temperature, with which we can calculate the heat flux using the formula: ![]() , where A is the surface of the black absorbing coating in m2.
, where A is the surface of the black absorbing coating in m2.
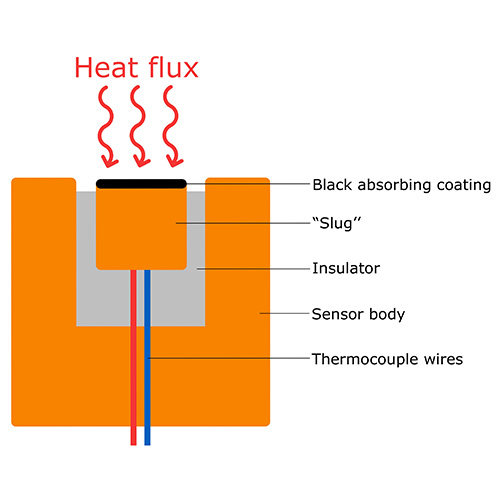
Often used in rocket propulsion heat flux measurements.
Slug calorimeters are used in spacecraft propulsion system measurements where they measure extreme heat flux for a short amount of time, often destroying (part of) the sensor. In this case, slug calorimeters are used, because they are cheaper to make than thermopile-based heat flux sensors.
The thermopile-based heat flux sensor
The type of heat flux sensors (heat flux transducers if we want to be technically correct) we make at Hukseflux are all based on thermopiles.
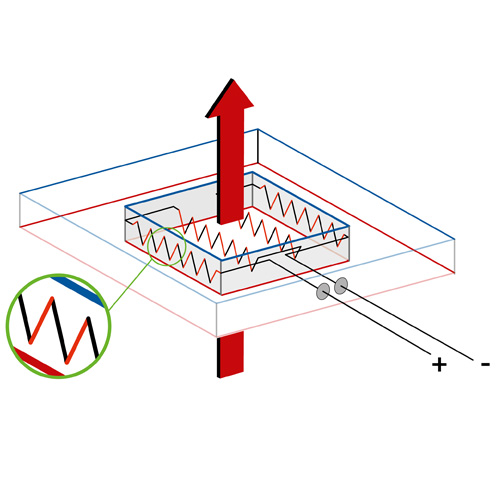
pattern of two dissimilar, conducting alloys or metals.
A thermopile, as seen in Figure 4, is made by connecting multiple thermocouples to each other. As mentioned before, a thermocouple is a sensor that generates a voltage when exposed to a temperature change. This occurs due to the interaction between two distinct metals or alloys that conduct electricity. The voltage is generated, because of the different reactions the materials have to the change in temperature. By calibrating the sensors, we can relate the output voltage to a temperature change, which gives us the sensor's sensitivity.
A more in-depth description of thermopiles can be found in our article: How to measure heat flux.
Thermopile-based heat flux transducers measure a constant heat flux, this is called “steady-state heat flux” and can be seen in Figure 5. In this case, the heat flux and output signal are constant, in contrast to calorimeters where a constant heat flux results in a constant temperature change over time.
One example of steady-state heat flux is determining the efficiency of insulation; one can measure steady-state heat flux as the heat that flows out of a house.
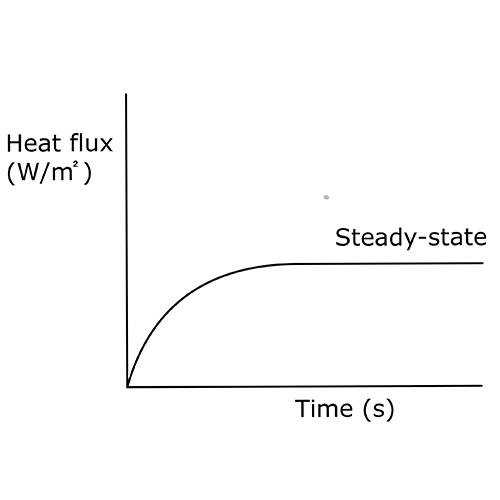
and steady-state heat flux.
Sensor or transducer?
So, why are transducers often called sensors? This is simply because not many people care about formalities, and “sensor” is a well-known and understood term while “transducer” is hardly used and often requires an explanation.
If you need a low-cost heat flux transducer then take a closer look at how calorimeters are made and try to make one yourself.
But if accurate, steady-state heat flux measurements need to be conducted, then take a look at our article: Which heat flux sensor to choose? or contact us at Hukseflux so we can help you find the right transducer for your needs.